How Do We Use Uranium
What is Uranium? How Does it Piece of work?
(Updated June 2022)
- Uranium is a heavy metallic which has been used every bit an arable source of full-bodied energy for over 60 years.
- Uranium occurs in most rocks in concentrations of 2 to 4 parts per 1000000 and is every bit common in the Earth'southward crust equally tin, tungsten and molybdenum. Uranium occurs in seawater, and can be recovered from the oceans.
- Uranium was discovered in 1789 by Martin Klaproth, a German language pharmacist, in the mineral chosen pitchblende. It was named later on the planet Uranus, which had been discovered eight years before.
- Uranium was patently formed in supernovae about 6.6 billion years ago. While it is non common in the solar system, today its deadening radioactivity provides the main source of heat inside the Earth, causing convection and continental migrate.
- The high density of uranium means that it besides finds uses in the keels of yachts and as counterweights for aircraft control surfaces, as well as for radiation shielding.
- Uranium has a melting signal of 1132°C. The chemical symbol for uranium is U.
The uranium atom
On a scale bundled according to the increasing mass of their nuclei, uranium is one of the heaviest of all the naturally-occurring elements (hydrogen is the lightest). Uranium is 18.7 times as dense every bit water.
Similar other elements, uranium occurs in several slightly differing forms known as 'isotopes'. These isotopes differ from each other in the number of uncharged particles (neutrons) in the nucleus. Natural uranium as found in the Earth's crust is a mixture largely of two isotopes: uranium-238 (U-238), accounting for 99.3% and uranium-235 (U-235) about 0.7%.
The isotope U-235 is important because under certain conditions it tin readily be split, yielding a lot of energy. It is therefore said to be 'fissile' and nosotros use the expression 'nuclear fission'.
Meanwhile, similar all radioactive isotopes, they decay. U-238 decays very slowly, its half-life being about the same equally the age of the Earth (4500 million years). This ways that it is barely radioactive, less so than many other isotopes in rocks and sand. Nonetheless information technology generates 0.1 watts/tonne as decay heat and this is enough to warm the World's core. U-235 decays slightly faster.
Free energy from the uranium atom
The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). When the nucleus of a U-235 atom captures a moving neutron it splits in two (fissions) and releases some energy in the form of oestrus, also two or three additional neutrons are thrown off. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, a fission 'chain reaction' can be achieved. When this happens over and over over again, many millions of times, a very large amount of oestrus is produced from a relatively small corporeality of uranium.
It is this process, in upshot 'burning' uranium, which occurs in a nuclear reactor. The heat is used to brand steam to produce electricity.
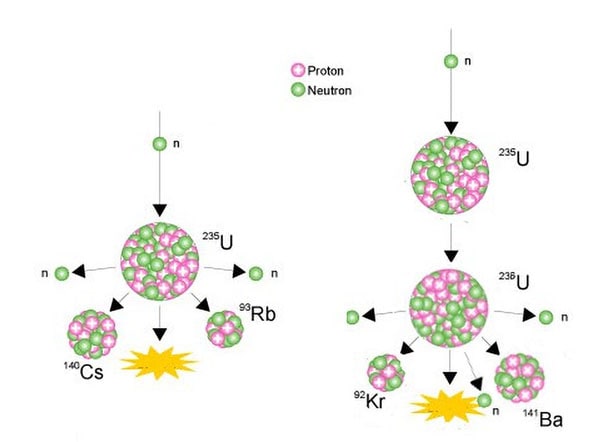
Examples of nuclear fissioning of uranium-235
Inside the reactor
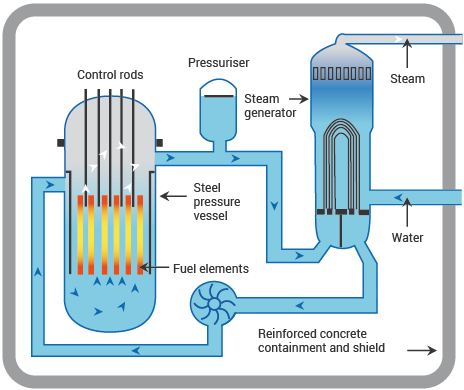
Nuclear power stations and fossil-fuelled power stations of like capacity have many features in common. Both require heat to produce steam to bulldoze turbines and generators. In a nuclear power station, yet, the fissioning of uranium atoms replaces the burning of coal or gas. In a nuclear reactor the uranium fuel is assembled in such a style that a controlled fission chain reaction can exist achieved. The heat created by splitting the U-235 atoms is then used to make steam which spins a turbine to drive a generator, producing electricity.
The concatenation reaction that takes place in the cadre of a nuclear reactor is controlled by rods which blot neutrons and which can be inserted or withdrawn to set the reactor at the required power level.
The fuel elements are surrounded by a substance called a moderator to slow the speed of the emitted neutrons and thus enable the chain reaction to go on. H2o, graphite and heavy water are used equally moderators in different types of reactor.
Because of the kind of fuel used (i.due east. the concentration of U-235, encounter beneath), if there is a major uncorrected malfunction in a reactor the fuel may overheat and melt, but information technology cannot explode like a flop.
A typical grand megawatt (MWe) reactor can provide enough electricity for a mod city of upwardly to 1 meg people.
Uranium and plutonium
Whereas the U-235 nucleus is 'fissile', that of U-238 is said to be 'fertile'. This ways that it tin capture one of the neutrons which are flight almost in the core of the reactor and become (indirectly) plutonium-239, which is fissile. Pu-239 is very much like U-235, in that it fissions when hit by a neutron and this yields a similar corporeality of energy.
Because there is then much U-238 in a reactor core (about of the fuel), these reactions occur oftentimes, and in fact about one-third of the fuel'southward energy yield comes from 'called-for' Pu-239.
But sometimes a Pu-239 atom but captures a neutron without splitting, and it becomes Pu-240. Because the Pu-239 is either progressively 'burned' or becomes Pu-240, the longer the fuel stays in the reactor the more Pu-240 is in it. (The significance of this is that when the spent fuel is removed after about three years, the plutonium in it is not suitable for making weapons but tin be recycled as fuel.)
From uranium ore to reactor fuel
Uranium ore tin can exist mined by clandestine or open-cut methods, depending on its depth. After mining, the ore is crushed and ground upwards. So it is treated with acrid to dissolve the uranium, which is recovered from solution.
Uranium may also exist mined by in situ leaching (ISL), where information technology is dissolved from a porous clandestine ore body in situ and pumped to the surface.
The end product of the mining and milling stages, or of ISL, is uranium oxide concentrate (U3O8). This is the form in which uranium is sold.
Earlier it can be used in a reactor for electricity generation, however, it must undergo a series of processes to produce a useable fuel.
For well-nigh of the world's reactors, the next pace in making the fuel is to catechumen the uranium oxide into a gas, uranium hexafluoride (UF6), which enables it to be enriched. Enrichment increases the proportion of the uranium-235 isotope from its natural level of 0.seven% to 4-v%. This enables greater technical efficiency in reactor blueprint and functioning, particularly in larger reactors, and allows the use of ordinary water equally a moderator.
After enrichment, the UF6 gas is converted to uranium dioxide (UO2) which is formed into fuel pellets. These fuel pellets are placed within thin metal tubes, known every bit fuel rods, which are assembled in bundles to become the fuel elements or assemblies for the core of the reactor. In a typical big ability reactor there might be 51,000 fuel rods with over 18 million pellets.

A worker holds up a newly made fuel pellet (KazAtomProm)
For reactors which use natural uranium equally their fuel (and hence which require graphite or heavy h2o as a moderator) the U3Oviii concentrate only needs to be refined and converted directly to uranium dioxide.
When the uranium fuel has been in the reactor for about three years, the used fuel is removed, stored, and so either reprocessed or disposed of underground (meet Nuclear Fuel Bike or Radioactive Waste Management).
Who uses nuclear ability?
About 10% of the world's electricity is generated from uranium in nuclear reactors. This amounts to over 2500 TWh each year, as much as from all sources of electricity worldwide in 1960.
It comes from about 440 nuclear reactors with a total output capacity of about 390,000 megawatts (MWe) operating in 32 countries. About 55 more reactors are under construction and about 100 are planned.
Belgium, Bulgaria, Czech Republic, Republic of finland, France, Hungary, Slovakia, Slovenia, Sweden, Switzerland and Ukraine all go xxx% or more than of their electricity from nuclear reactors. The United states has about xc reactors operating, supplying 20% of its electricity. France gets over 70% of its electricity from uranium.
Over the 60 years that the earth has enjoyed the benefits of cleanly-generated electricity from nuclear power, there have been over 18,000 reactor-years of operational experience.
See besides Nuclear Generation by Country.
Who has and who mines uranium?
Uranium is widespread in many rocks, and fifty-fifty in seawater. However, similar other metals, it is seldom sufficiently concentrated to exist economically recoverable. Where it is, we speak of an orebody. In defining what is ore, assumptions are made nigh the cost of mining and the market toll of the metal. Uranium reserves are therefore calculated as tonnes recoverable up to a sure cost.
Uranium resources past state in 2019
| tonnes U | percentage of world | |
| Commonwealth of australia | 1,692,700 | 28% |
|---|---|---|
| Kazakhstan | 906,800 | fifteen% |
| Canada | 564,900 | nine% |
| Russia | 486,000 | 8% |
| Namibia | 448,300 | seven% |
| Due south Africa | 320,900 | 5% |
| Brazil | 276,800 | v% |
| Niger | 276,400 | 4% |
| China | 248,900 | 4% |
| Mongolia | 143,500 | ii% |
| Uzbekistan | 132,300 | ii% |
| Ukraine | 108,700 | ii% |
| Botswana | 87,200 | 1% |
| Tanzania | 58,200 | i% |
| Jordan | 52,500 | 1% |
| USA | 47,900 | i% |
| Other | 295,800 | 5% |
| World total | 6,147,800 |
Identified resources recoverable (reasonably assured resource plus inferred resources), to $130/kg U, 1/1/19, from OECD NEA & IAEA,Uranium 2020: Resources, Production and Need ('Red Book'). The full recoverable identified resources to $260/kg U is 8.070 million tonnes U.
Product from mines (tonnes U)
| Country | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Kazakhstan | 21,317 | 22,451 | 23,127 | 23,607 | 24,689 | 23,321 | 21,705 | 22,808 | 19,477 | 21,819 |
|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 6991 | 6350 | 5001 | 5654 | 6315 | 5882 | 6517 | 6613 | 6203 | 4192 |
| Namibia | 4495 | 4323 | 3255 | 2993 | 3654 | 4224 | 5525 | 5476 | 5413 | 5753 |
| Canada | 8999 | 9331 | 9134 | 13,325 | 14,039 | xiii,116 | 7001 | 6938 | 3885 | 4693 |
| Uzbekistan (est.) | 2400 | 2400 | 2400 | 2385 | 3325 | 3400 | 3450 | 3500 | 3500 | 3500 |
| Niger | 4667 | 4518 | 4057 | 4116 | 3479 | 3449 | 2911 | 2983 | 2991 | 2248 |
| Russia | 2872 | 3135 | 2990 | 3055 | 3004 | 2917 | 2904 | 2911 | 2846 | 2635 |
| China (est.) | 1500 | 1500 | 1500 | 1616 | 1616 | 1692 | 1885 | 1885 | 1885 | 1885 |
| Ukraine | 960 | 922 | 926 | 1200 | 808 | 707 | 790 | 800 | 744 | 455 |
| India (est.) | 385 | 385 | 385 | 385 | 385 | 421 | 423 | 308 | 400 | 615 |
| South Africa (est.) | 465 | 531 | 573 | 393 | 490 | 308 | 346 | 346 | 250 | 385 |
| Islamic republic of iran (est.) | 0 | 0 | 0 | 38 | 0 | forty | 71 | 71 | 71 | 71 |
| Pakistan (est.) | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| USA | 1596 | 1792 | 1919 | 1256 | 1125 | 940 | 582 | 58 | vi | 8 |
| Brazil | 326 | 192 | 55 | twoscore | 44 | 0 | 0 | 0 | fifteen | 0 |
| Czech republic | 228 | 215 | 193 | 155 | 138 | 0 | 0 | 0 | 0 | 0 |
| Romania | xc | 77 | 77 | 77 | fifty | 0 | 0 | 0 | 0 | 0 |
| France | iii | v | 3 | ii | 0 | 0 | 0 | 0 | 0 | 0 |
| Deutschland | fifty | 27 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malawi | 1101 | 1132 | 369 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total world | 58,493 | 59,331 | 56,041 | sixty,304 | 63,207 | lx,514 | 54,154 | 54,742 | 47,731 | 48,303 |
| tonnes U3Oeight | 68,974 | 69,966 | 66,087 | 71,113 | 74,357 | 71,361 | 63,861 | 64,554 | 56,287 | 56,961 |
| % of world demand | 94% | 91% | 85% | 98% | 96% | 93% | 80% | 81% | 74% | 77% |
* Information from the World Nuclear Association. NB: the figures in this tabular array are liable to change as new data becomes available. Totals may not sum exactly due to rounding.
Mining methods have been changing. In 1990, 55% of globe product came from underground mines, merely this shrunk dramatically to 1999, with 33% then. From 2000 the new Canadian mines increased it again. In situ leach (ISL, also called in situ recovery, ISR) mining has been steadily increasing its share of the total, mainly due to Kazakhstan, and in 2021 accounted for over threescore% of production:
| Method | tonnes U | % |
| In situ leach (ISL) | 32,088 | 66% |
| Underground & open pit (except Olympic Dam) | 13908 | 29% |
| By-production | 2307 | 5% |
Other uses of nuclear energy
Uranium is sold only to countries which are signatories of the Nuclear Non-Proliferation Treaty (NPT), and which allow international inspection to verify that it is used only for peaceful purposes.
Many people, when talking about nuclear energy, have only nuclear reactors (or peradventure nuclear weapons) in mind. Few people realise the extent to which the utilize of radioisotopes has changed our lives over the last few decades.
Using relatively pocket-sized special-purpose nuclear reactors, it is possible to make a wide range of radioactive materials (radioisotopes) at low toll. For this reason the use of artificially-produced radioisotopes has go widespread since the early 1950s, and there are now nigh 220 'research' reactors in 56 countries producing them. These are substantially neutron factories rather than sources of heat.
Radioisotopes
In our daily life we demand nutrient, water and good health. Today, radioactive isotopes play an important part in the technologies that provide usa with all three. They are produced by bombarding minor amounts of particular elements with neutrons.
Inmedicine, radioisotopes are widely used for diagnosis and research. Radioactive chemic tracers emit gamma radiation which provides diagnostic information about a person's anatomy and the performance of specific organs. Radiotherapy also employs radioisotopes in the treatment of some illnesses, such equally cancer. About one person in two in the Western earth is probable to feel the benefits of nuclear medicine in their lifetime. More powerful gamma sources are used to sterilise syringes, bandages and other medical utensils – gamma sterilisation of equipment is nigh universal.
In thepreservation of food, radioisotopes are used to inhibit the sprouting of root crops after harvesting, to impale parasites and pests, and to control the ripening of stored fruit and vegetables. Irradiated foodstuffs are accepted by world and national health authorities for human consumption in an increasing number of countries. They include potatoes, onions, dried and fresh fruits, grain and grain products, poultry and some fish. Some prepacked foods can also exist irradiated.
In the growing of crops and breedinglivestock, radioisotopes also play an important role. They are used to produce high yielding, affliction-resistant and weather-resistant varieties of crops, to study how fertilisers and insecticides work, and to improve the productivity and health of domestic animals.
Industrially, and in mining, they are used to examine welds, to detect leaks, to study the rate of clothing of metals, and for on-stream analysis of a broad range of minerals and fuels.
There are many other uses. A radioisotope derived from the plutonium formed in nuclear reactors is used in virtually householdfume detectors.
Radioisotopes are used to detect and analyse pollutants in the surroundings, and to study the movement of surface water in streams and also of groundwater.
See also The Many Uses of Nuclear Applied science.
Other reactors
There are likewise other uses for nuclear reactors. Near 200 small-scale nuclear reactors power some 150 ships, mostly submarines, only ranging from icebreakers to aircraft carriers. These can stay at ocean for long periods without having to make refuelling stops. In the Russian Arctic where operating weather are beyond the adequacy of conventional icebreakers, very powerful nuclear-powered vessels operate year-circular, where previously only ii months allowed northern access each year.
The heat produced by nuclear reactors tin also be used direct rather than for generating electricity. In Sweden, Russian federation and China, for example, surplus oestrus is used to oestrus buildings. Nuclear oestrus may besides be used for a variety of industrial processes such as water desalination. Nuclear desalination is likely to be a major growth area in the adjacent decade.
High-temperature estrus from nuclear reactors is likely to be employed in some industrial processes in future, especially for making hydrogen.
Armed forces sources of fuel
Both uranium and plutonium were used to make bombs earlier they became important for making electricity and radioisotopes. The type of uranium and plutonium for bombs is different from that in a nuclear power plant. Bomb-grade uranium is highly-enriched (>xc% U-235, instead of upward to v%); bomb-grade plutonium is fairly pure Pu-239 (>90%, instead of almost sixty% in reactor-grade) and is made in special reactors.
Since the 1990s, due to disarmament, a lot of military uranium has become available for electricity production. The military uranium is diluted about 25:1 with depleted uranium (more often than not U-238) from the enrichment process before being used in power generation. Over 2 decades to 2013 ane-tenth of U.s. electricity was made from Russian weapons uranium.
How Do We Use Uranium,
Source: https://world-nuclear.org/information-library/nuclear-fuel-cycle/introduction/what-is-uranium-how-does-it-work.aspx
Posted by: randallhatione.blogspot.com


0 Response to "How Do We Use Uranium"
Post a Comment