Used To Measure Air Pressure

A barometer is a scientific musical instrument that is used to measure out air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface conditions analysis to assistance discover surface troughs, pressure systems and frontal boundaries.
Barometers and pressure altimeters (the nearly basic and common type of altimeter) are essentially the same instrument, but used for dissimilar purposes. An altimeter is intended to be used at different levels matching the corresponding atmospheric pressure to the altitude, while a barometer is kept at the aforementioned level and measures subtle force per unit area changes acquired by weather and elements of weather. The boilerplate atmospheric pressure on the earth'south surface varies between 940 and 1040 hPa (mbar). The boilerplate atmospheric pressure at sea level is 1013 hPa (mbar).
Etymology
The give-and-take barometer is derived from the Ancient Greek βάρος ( báros ), pregnant "weight", and μέτρον ( métron ), meaning "measure".
History
Although Evangelista Torricelli is universally credited with inventing the barometer in 1643,[1] [ii] historical documentation too suggests Gasparo Berti, an Italian mathematician and astronomer, unintentionally built a h2o barometer erstwhile between 1640 and 1643.[1] [iii] French scientist and philosopher René Descartes described the blueprint of an experiment to determine atmospheric pressure as early on as 1631, but there is no show that he built a working barometer at that fourth dimension.[1]
On 27 July 1630, Giovanni Battista Baliani wrote a letter to Galileo Galilei explaining an experiment he had made in which a siphon, led over a hill virtually twenty-one meters high, failed to work. Galileo responded with an caption of the phenomenon: he proposed that it was the power of a vacuum that held the water upward, and at a certain top the amount of h2o just became too much and the forcefulness could not agree any more than, similar a cord that can back up only so much weight.[4] [5] This was a restatement of the theory of horror vacui ("nature abhors a vacuum"), which dates to Aristotle, and which Galileo restated as resistenza del vacuo.
Galileo's ideas reached Rome in December 1638 in his Discorsi. Raffaele Magiotti and Gasparo Berti were excited by these ideas, and decided to seek a better way to endeavour to produce a vacuum other than with a siphon. Magiotti devised such an experiment, and erstwhile betwixt 1639 and 1641, Berti (with Magiotti, Athanasius Kircher and Niccolò Zucchi present) carried it out.[5]
Four accounts of Berti'south experiment exist, simply a simple model of his experiment consisted of filling with water a long tube that had both ends plugged, then continuing the tube in a bowl already full of water. The bottom end of the tube was opened, and h2o that had been inside of it poured out into the basin. Still, but function of the h2o in the tube flowed out, and the level of the h2o inside the tube stayed at an exact level, which happened to be 10.3 m (34 ft),[half-dozen] the same height Baliani and Galileo had observed that was limited by the siphon. What was virtually important about this experiment was that the lowering water had left a infinite higher up it in the tube which had no intermediate contact with air to fill it upwardly. This seemed to suggest the possibility of a vacuum existing in the space above the water.[v]
Torricelli, a friend and student of Galileo, interpreted the results of the experiments in a novel way. He proposed that the weight of the atmosphere, not an attracting force of the vacuum, held the water in the tube. In a letter to Michelangelo Ricci in 1644 apropos the experiments, he wrote:
Many have said that a vacuum does not exist, others that it does be in spite of the repugnance of nature and with difficulty; I know of no one who has said that information technology exists without difficulty and without a resistance from nature. I argued thus: If in that location tin can exist constitute a manifest crusade from which the resistance can exist derived which is felt if we try to brand a vacuum, it seems to me foolish to endeavour to attribute to vacuum those operations which follow evidently from some other cause; and so by making some very easy calculations, I found that the crusade assigned by me (that is, the weight of the atmosphere) ought by itself alone to offer a greater resistance than it does when we attempt to produce a vacuum.[vii]
It was traditionally thought (peculiarly past the Aristotelians) that the air did non take weight: that is, that the kilometers of air above the surface did not exert whatsoever weight on the bodies below it. Even Galileo had accepted the weightlessness of air as a uncomplicated truth. Torricelli questioned that assumption, and instead proposed that air had weight and that it was the latter (not the attracting forcefulness of the vacuum) which held (or rather, pushed) up the column of water. He thought that the level the water stayed at (c. 10.3 m) was reflective of the force of the air's weight pushing on it (specifically, pushing on the water in the basin and thus limiting how much water tin can fall from the tube into it). In other words, he viewed the barometer every bit a residue, an musical instrument for measurement (as opposed to merely existence an instrument to create a vacuum), and considering he was the first to view it this way, he is traditionally considered the inventor of the barometer (in the sense in which we at present use the term).[5]
Considering of rumors circulating in Torricelli'southward gossipy Italian neighbourhood, which included that he was engaged in some form of sorcery or witchcraft, Torricelli realized he had to keep his experiment secret to avoid the risk of beingness arrested. He needed to use a liquid that was heavier than h2o, and from his previous association and suggestions by Galileo, he deduced that past using mercury, a shorter tube could be used. With mercury, which is about 14 times denser than water, a tube only fourscore cm was now needed, not 10.5 m.[viii]
In 1646, Blaise Pascal forth with Pierre Petit, had repeated and perfected Torricelli's experiment subsequently hearing about it from Marin Mersenne, who himself had been shown the experiment by Torricelli toward the stop of 1644. Pascal farther devised an experiment to test the Aristotelian proposition that information technology was vapours from the liquid that filled the space in a barometer. His experiment compared water with vino, and since the latter was considered more "spiritous", the Aristotelians expected the vino to stand lower (since more vapours would mean more pushing downwards on the liquid cavalcade). Pascal performed the experiment publicly, inviting the Aristotelians to predict the outcome beforehand. The Aristotelians predicted the vino would stand lower. It did non.[v]
However, Pascal went fifty-fifty further to examination the mechanical theory. If, as suspected by mechanical philosophers similar Torricelli and Pascal, air had weight, the pressure would be less at college altitudes. Therefore, Pascal wrote to his brother-in-constabulary, Florin Perier, who lived about a mountain called the Puy de Dôme, asking him to perform a crucial experiment. Perier was to take a barometer upwards the Puy de Dôme and brand measurements forth the way of the elevation of the column of mercury. He was and so to compare it to measurements taken at the pes of the mount to see if those measurements taken higher up were in fact smaller. In September 1648, Perier carefully and meticulously carried out the experiment, and plant that Pascal's predictions had been right. The mercury barometer stood lower the college one went.[5]
Types
Water barometers

The concept that decreasing atmospheric force per unit area predicts stormy atmospheric condition, postulated by Lucien Vidi, provides the theoretical basis for a weather prediction device chosen a "weather condition glass" or a "Goethe barometer" (named for Johann Wolfgang von Goethe, the renowned German writer and polymath who adult a simple just effective weather ball barometer using the principles developed past Torricelli). The French name, le baromètre Liègeois, is used by some English speakers.[9] This name reflects the origins of many early weather glasses – the glass blowers of Liège, Belgium.[ix] [10]
The weather ball barometer consists of a glass container with a sealed body, half filled with water. A narrow spout connects to the body below the h2o level and rises above the water level. The narrow spout is open to the atmosphere. When the air force per unit area is lower than information technology was at the time the body was sealed, the water level in the spout will rise above the h2o level in the body; when the air pressure is higher, the water level in the spout will drop below the water level in the body. A variation of this type of barometer can exist hands made at abode.[11]
Mercury barometers
A mercury barometer is an instrument used to mensurate atmospheric pressure in a certain location and has a vertical drinking glass tube closed at the top sitting in an open mercury-filled basin at the lesser. Mercury in the tube adjusts until the weight of it balances the atmospheric forcefulness exerted on the reservoir. High atmospheric pressure places more strength on the reservoir, forcing mercury higher in the cavalcade. Low pressure allows the mercury to drop to a lower level in the column past lowering the force placed on the reservoir. Since college temperature levels around the musical instrument will reduce the density of the mercury, the scale for reading the peak of the mercury is adjusted to compensate for this effect. The tube has to be at to the lowest degree as long as the amount dipping in the mercury + head space + the maximum length of the column.
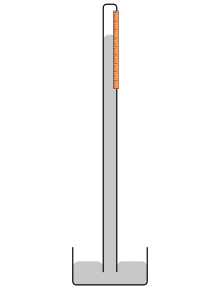
Schematic drawing of a simple mercury barometer with vertical mercury column and reservoir at base of operations
Torricelli documented that the height of the mercury in a barometer inverse slightly each day and concluded that this was due to the irresolute pressure level in the atmosphere.[ane] He wrote: "We live submerged at the lesser of an ocean of uncomplicated air, which is known by incontestable experiments to accept weight".[12] Inspired by Torricelli, Otto von Guericke on v December 1660 found that air pressure was unusually low and predicted a tempest, which occurred the next day.[thirteen]
The mercury barometer'due south design gives rise to the expression of atmospheric pressure in inches or millimeters of mercury (mmHg). A torr was originally divers every bit 1 mmHg. The pressure is quoted as the level of the mercury'southward pinnacle in the vertical column. Typically, atmospheric pressure is measured between 26.5 inches (670 mm) and 31.5 inches (800 mm) of Hg. One atmosphere (i atm) is equivalent to 29.92 inches (760 mm) of mercury.
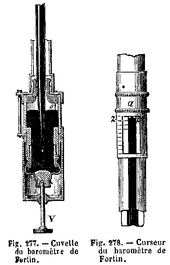
Reservoir of a Fortin barometer
Pattern changes to make the musical instrument more sensitive, simpler to read, and easier to transport resulted in variations such as the bowl, siphon, wheel, cistern, Fortin, multiple folded, stereometric, and balance barometers.
In 2007, a European Union directive was enacted to restrict the utilise of mercury in new measuring instruments intended for the full general public, effectively ending the production of new mercury barometers in Europe. The repair and trade of antiques (produced before late 1957) remained unrestricted.[14] [xv]
Fitzroy barometer
Fitzroy barometers combine the standard mercury barometer with a thermometer, equally well as a guide of how to interpret force per unit area changes.

Sympiesometer inscribed at bottom Improved sympiesometer and at top A R Easton, 53 Marischal Street, Aberdeen. Owned by descendants of the Aberdeen shipbuilding Hall family unit.
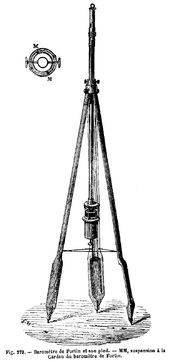
Fortin barometer
Fortin barometers use a variable displacement mercury cistern, unremarkably constructed with a thumbscrew pressing on a leather diaphragm bottom (V in the diagram). This compensates for displacement of mercury in the column with varying pressure. To use a Fortin barometer, the level of mercury is prepare to null by using the thumbscrew to make an ivory arrow (O in the diagram) simply bear upon the surface of the mercury. The pressure is so read on the column past adjusting the vernier scale then that the mercury only touches the sightline at Z. Some models as well employ a valve for endmost the cistern, enabling the mercury column to be forced to the top of the cavalcade for ship. This prevents water-hammer impairment to the cavalcade in transit.
Sympiesometer
A sympiesometer is a compact and lightweight barometer that was widely used on ships in the early on 19th century. The sensitivity of this barometer was also used to measure altitude.[16]
Sympiesometers have 2 parts. One is a traditional mercury thermometer that is needed to calculate the expansion or contraction of the fluid in the barometer. The other is the barometer, consisting of a J-shaped tube open at the lower end and closed at the top, with small reservoirs at both ends of the tube.
Bicycle barometers
A wheel barometer uses a "J" tube sealed at the pinnacle of the longer limb. The shorter limb is open up to the atmosphere and floating on meridian of the mercury there is a small glass float. A fine silken thread is fastened to the float which passes up over a wheel and so back downwards to a counterweight (usually protected in some other tube). The wheel turns the signal on the front of the barometer. As atmospheric pressure increases mercury moves from the brusque to the long limb, the float falls and the pointer moves. When pressure level increases the mercury moves dorsum, lifting the float and turning the dial the other way.[17]
Effectually 1810 the wheel barometer, which could be read from a corking distance, became the get-go practical and commercial instrument favoured by farmers and the educated classes in the United kingdom. The confront of the barometer was circular with a simple dial pointing to an hands readable calibration: "Rain - Change - Dry out" with the "Alter" at the pinnacle centre of the dial. Later models added a barometric scale with finer graduations "Stormy (28 inches of mercury), Much Rain (28.5), Rain (29), Change (29.5), Fair (30), Set off-white (30.5), very dry(31)".
Natalo Aiano is recognised as ane of the finest makers of cycle barometers, an early pioneer in a moving ridge of artisanal Italian musical instrument and barometer makers that were encouraged to emigrate to the United kingdom of great britain and northern ireland. He listed as working in Holborn, London c.1785-1805.[xviii] From 1770 onwards a big number of Italians came to England because they were accomplished glass blowers or instrument makers. By 1840 it was fair to say that the Italians dominated the industry in England.[nineteen]
Vacuum pump oil barometer
Using vacuum pump oil equally the working fluid in a barometer has led to the cosmos of the new "World's Tallest Barometer" in Feb 2013. The barometer at Portland State University (PSU) uses doubly distilled vacuum pump oil and has a nominal height of about 12.four chiliad for the oil column tiptop; expected excursions are in the range of ±0.iv m over the course of a year. Vacuum pump oil has very depression vapour pressure and it is available in a range of densities; the lowest density vacuum oil was called for the PSU barometer to maximize the oil cavalcade summit.[twenty]
Aneroid barometers

An aneroid barometer is an instrument used for measuring air force per unit area as a method that does not involve liquid. Invented in 1844 past French scientist Lucien Vidi,[21] the aneroid barometer uses a small, flexible metal box called an aneroid prison cell (sheathing), which is made from an alloy of beryllium and copper. The evacuated capsule (or commonly several capsules, stacked to add upward their movements) is prevented from collapsing by a strong spring. Pocket-size changes in external air force per unit area cause the cell to expand or contract. This expansion and contraction drives mechanical levers such that the tiny movements of the capsule are amplified and displayed on the face of the aneroid barometer. Many models include a manually set needle which is used to mark the electric current measurement so a alter can be seen. This blazon of barometer is mutual in homes and in recreational boats. It is besides used in meteorology, mostly in barographs and as a pressure instrument in radiosondes.
Barographs
A barograph is a recording aneroid barometer where the changes in atmospheric pressure are recorded on a newspaper nautical chart.
The principle of the barograph is same every bit that of the aneroid barometer. Whereas the barometer displays the pressure on a dial, the barograph uses the small movements of the box to transmit past a system of levers to a recording arm that has at its extreme end either a scribe or a pen. A scribe records on smoked foil while a pen records on paper using ink, held in a pecker. The recording material is mounted on a cylindrical pulsate which is rotated slowly by a clock. Commonly, the drum makes 1 revolution per day, per calendar week, or per month and the rotation rate can often exist selected by the user.
MEMS barometers

Microelectromechanical systems (or MEMS) barometers are extremely small devices between 1 and 100 micrometres in size (0.001 to 0.1 mm). They are created via photolithography or photochemical machining. Typical applications include miniaturized weather stations, electronic barometers and altimeters.[22]
A barometer can also be constitute in smartphones such as the Samsung Galaxy Nexus,[23] Samsung Galaxy S3-S6, Motorola Xoom, Apple iPhone six and newer iPhones, and Timex Expedition WS4 smartwatch, based on MEMS and piezoresistive force per unit area-sensing technologies.[24] [25] Inclusion of barometers on smartphones was originally intended to provide a faster GPS lock.[26] Nonetheless, third party researchers were unable to ostend boosted GPS accurateness or lock speed due to barometric readings. The researchers suggest that the inclusion of barometers in smartphones may provide a solution for determining a user's elevation, simply also suggest that several pitfalls must first be overcome.[27]
More than unusual barometers

Timex Expedition WS4 in Barometric chart mode with atmospheric condition forecast function
There are many other more unusual types of barometer. From variations on the storm barometer, such as the Collins Patent Table Barometer, to more traditional-looking designs such every bit Hooke'due south Otheometer and the Ross Sympiesometer. Some, such as the Shark Oil barometer,[28] work only in a certain temperature range, accomplished in warmer climates.
Applications

Digital graphing barometer.

Analogue recording barograph using 5 stacked aneroid barometer cells.
Barometric pressure level and the pressure tendency (the change of pressure over fourth dimension) accept been used in atmospheric condition forecasting since the belatedly 19th century.[29] When used in combination with wind observations, reasonably authentic short-term forecasts tin can be made.[thirty] Simultaneous barometric readings from across a network of weather condition stations allow maps of air pressure to be produced, which were the first class of the mod weather map when created in the 19th century. Isobars, lines of equal pressure, when drawn on such a map, give a contour map showing areas of high and low pressure.[31] Localized high atmospheric force per unit area acts as a bulwark to budgeted atmospheric condition systems, diverting their course. Atmospheric elevator caused by low-level current of air convergence into the surface brings clouds and sometimes precipitation.[32] The larger the change in pressure, especially if more than 3.5 hPa (0.1 inHg), the greater the modify in weather that can exist expected. If the pressure drib is rapid, a depression pressure system is approaching, and there is a greater chance of rain. Rapid pressure rises, such as in the wake of a cold forepart, are associated with improving weather conditions, such as clearing skies.[33]
With falling air pressure, gases trapped within the coal in deep mines can escape more than freely. Thus depression pressure increases the risk of firedamp accumulating. Collieries therefore keep track of the pressure. In the instance of the Trimdon Grange colliery disaster of 1882 the mines inspector drew attention to the records and in the report stated "the conditions of temper and temperature may be taken to have reached a dangerous point".[34]
Aneroid barometers are used in scuba diving. A submersible pressure estimate is used to keep rail of the contents of the diver's air tank. Another gauge is used to measure the hydrostatic pressure, usually expressed every bit a depth of sea water. Either or both gauges may be replaced with electronic variants or a dive reckoner.[35]
Compensations
Temperature
The density of mercury will change with increment or subtract in temperature, so a reading must be adjusted for the temperature of the instrument. For this purpose a mercury thermometer is usually mounted on the instrument. Temperature compensation of an aneroid barometer is accomplished past including a bi-metal element in the mechanical linkages. Aneroid barometers sold for domestic use typically have no compensation under the supposition that they will be used inside a controlled room temperature range.
Distance

A digital barometer with altimeter setting (for correction) displayed
As the air pressure decreases at altitudes above sea level (and increases below bounding main level) the uncorrected reading of the barometer volition depend on its location. The reading is then adjusted to an equivalent ocean-level pressure for purposes of reporting. For case, if a barometer located at body of water level and under fair weather condition conditions is moved to an altitude of 1,000 feet (305 k), nigh 1 inch of mercury (~35 hPa) must exist added on to the reading. The barometer readings at the two locations should be the aforementioned if there are negligible changes in time, horizontal altitude, and temperature. If this were not done, at that place would exist a false indication of an approaching storm at the higher acme.
Aneroid barometers accept a mechanical adjustment that allows the equivalent body of water level pressure to be read directly and without further adjustment if the instrument is not moved to a dissimilar distance. Setting an aneroid barometer is similar to resetting an analog clock that is non at the right time. Its punch is rotated so that the current atmospheric pressure from a known accurate and nearby barometer (such as the local conditions station) is displayed. No adding is needed, every bit the source barometer reading has already been converted to equivalent sea-level force per unit area, and this is transferred to the barometer being fix—regardless of its altitude. Though somewhat rare, a few aneroid barometers intended for monitoring the conditions are calibrated to manually adjust for altitude. In this example, knowing either the altitude or the electric current atmospheric pressure would be sufficient for future accurate readings.
The tabular array beneath shows examples for three locations in the urban center of San Francisco, California. Note the corrected barometer readings are identical, and based on equivalent ocean-level pressure. (Presume a temperature of 15 °C.)
| Location | Distance (feet) | Uncorrected Patm (inches Hg) | Corrected Patm (inches Hg) | Distance (metres) | Uncorrected Patm (hPa) | Corrected Patm (hPa) | |
|---|---|---|---|---|---|---|---|
| City Marina | Sea Level (0) | 29.92 | 29.92 | 0 m | 1013 hPa | 1013 hPa | |
| Nob Colina | 348 | 29.55 | 29.92 | 106 one thousand | 1001 hPa | 1013 hPa | |
| Mt. Davidson | 928 | 28.94 | 29.92 | 283 m | 980 hPa | 1013 hPa |
In 1787, during a scientific expedition on Mont Blanc, De Saussure undertook enquiry and executed concrete experiments on the boiling betoken of water at different heights. He calculated the meridian at each of his experiments past measuring how long it took an booze burner to boil an amount of water, and by these means he determined the tiptop of the mountain to be 4775 metres. (This after turned out to exist 32 metres less than the actual height of 4807 metres). For these experiments De Saussure brought specific scientific equipment, such as a barometer and thermometer. His calculated humid temperature of water at the acme of the mount was adequately accurate, only off by 0.one kelvin.[36]
Based on his findings, the altimeter could be developed equally a specific application of the barometer. In the mid-19th century, this method was used by explorers.[37]
Equation
When atmospheric pressure level is measured by a barometer, the pressure is too referred to every bit the "barometric pressure". Assume a barometer with a cantankerous-sectional surface area A, a height h, filled with mercury from the bottom at Bespeak B to the top at Indicate C. The pressure at the bottom of the barometer, Indicate B, is equal to the atmospheric pressure. The pressure at the very peak, Bespeak C, tin can be taken as zero considering at that place is only mercury vapour above this point and its pressure is very depression relative to the atmospheric pressure. Therefore, ane can notice the atmospheric pressure using the barometer and this equation:[38] [ clarification needed ]
- Patm = ρgh
where ρ is the density of mercury, g is the gravitational dispatch, and h is the pinnacle of the mercury column above the complimentary area. The physical dimensions (length of tube and cross-exclusive area of the tube) of the barometer itself have no result on the tiptop of the fluid column in the tube.
In thermodynamic calculations, a unremarkably used pressure unit is the "standard temper". This is the pressure resulting from a column of mercury of 760 mm in top at 0 °C. For the density of mercury, employ ρHg = xiii,595 kg/m3 and for gravitational acceleration utilize g = 9.807 yard/stwo.
If water were used (instead of mercury) to meet the standard atmospheric pressure, a water cavalcade of roughly 10.3 m (33.8 ft) would be needed.
Standard atmospheric pressure as a office of meridian:
Note: 1 torr = 133.iii Pa = 0.03937 inHg
| Patm / kPa | Altitude | Patm / inHg | Distance | |
|---|---|---|---|---|
| 101.325 | Sea Level (0m) | 29.92 | Sea Level (0 ft) | |
| 97.71 | 305 m | 28.86 | one,000 ft | |
| 94.21 | 610 thousand | 27.82 | ii,000 ft | |
| 89.88 | 1,000 m | 26.55 | iii,281 ft | |
| 84.31 | 1,524 chiliad | 24.90 | 5,000 ft | |
| 79.l | ii,000 m | 23.48 | six,562 ft | |
| 69.68 | 3,048 m | 20.58 | 10,000 ft | |
| 54.05 | 5,000 grand | 15.96 | sixteen,404 ft | |
| 46.56 | 6,096 m | thirteen.75 | xx,000 ft | |
| 37.65 | 7,620 m | 11.12 | 25,000 ft | |
| 32.77 | 8,848 g* | 9.68 | 29,029 ft* | |
| 26.44 | x,000 m | 7.81 | 32,808 ft | |
| eleven.65 | 15,240 m | 3.44 | 50,000 ft | |
| v.53 | xx,000 m | one.63 | 65,617 ft |
- Elevation of Mount Everest, the highest indicate on earth
Patents

Table of Pneumaticks, 1728 Cyclopaedia
- The states 2194624, K. A. Titterington, Jr, "Diaphragm pressure gauge having temperature compensating means", issued 1940-03-26, assigned to Bendix Aviat Corp
- U.Southward. Patent 2,472,735 : C. J. Ulrich : "Barometric instrument"
- U.S. Patent 2,691,305 : H. J. Frank : "Barometric altimeter"
- U.S. Patent iii,273,398 : D. C. W. T. Sharp : "Aneroid barometer"
- U.S. Patent 3,397,578 : H. A. Klumb : "Motion amplifying mechanism for pressure level responsive instrument motion"
- U.S. Patent 3,643,510 : F. Lissau : "Fluid displacement pressure gauges"
- U.Due south. Patent 4,106,342 : O. S. Sormunen : "Pressure measuring musical instrument"
- U.S. Patent four,238,958 : H. Dostmann : "Barometer"
- U.S. Patent iv,327,583 : T. Fijimoto : "Weather forecasting device"
See also
- Altimeter
- Anemoscope
- Automated drome weather station
- Barograph
- Barometer question
- Bert Bolle Barometer
- Microbarometer
- Storm glass
- Surface weather analysis
- Tempest prognosticator
- Units of pressure
- Pressure sensor
- Weather forecasting
- Zambretti Forecaster
References
- ^ a b c d Heidorn, Keith C. (ane January 2002). "The Invention of the Barometer". Islandnet.com. Archived from the original on 14 May 2011. Retrieved four February 2010.
- ^ "History of the Barometer". Barometerfair.com. Archived from the original on 2009-09-25. Retrieved 2010-02-04 .
- ^ Drake, Stillman (1970). "Berti, Gasparo". Dictionary of Scientific Biography. Vol. 2. New York: Charles Scribner's Sons. pp. 83–84. ISBN978-0-684-10114-nine.
- ^ Shea, William R. (2003). Designing Experiments & Games of Chance: The Unconventional Scientific discipline of Blaise Pascal. Science History Publications. pp. 21–. ISBN978-0-88135-376-1 . Retrieved ten Oct 2012.
- ^ a b c d e f "History of the Barometer". Strange-loops.com. 2002-01-21. Archived from the original on vi January 2010. Retrieved 2010-02-04 .
- ^ Gillispie, Charles Coulston (1960). The Border of Objectivity: An Essay in the History of Scientific Ideas . Princeton University Press. pp. 99–100. ISBN0-691-02350-six.
- ^ "Torricelli'south alphabetic character to Michelangelo Ricci". Spider web.lemoyne.edu. Retrieved 2010-02-04 .
- ^ "Brief History of the Barometer". Barometer.ws. Archived from the original on 14 Jan 2010. Retrieved 2010-02-04 .
- ^ a b Gerard L'E. Turner, Nineteenth Century Scientific Instruments, Sotheby Publications, 1983, p 236, ISBN 0-85667-170-3
- ^ Claus Zittle, Philosophies of Technology: Francis Salary and His Contemporaries, BRILL 2008, pp 115, 116 ISBN 90-04-17050-ii
- ^ Jet Stream. Learning Lesson: Measure the Pressure – The "Moisture" Barometer. Retrieved on 2019-01-21.
- ^ Strangeways, Ian. Measuring the Natural Environment. Cambridge Academy Press, 2000, p. 92.
- ^ Ley, Willy (June 1966). "The Re-Designed Solar System". For Your Information. Galaxy Science Fiction. pp. 94–106.
- ^ Jones H. (ten July 2007). "Eu bans mercury in barometers, thermometers". Reuters . Retrieved 12 September 2017.
- ^ "Ban on auction of mercury measuring instruments - MEPs concur two twelvemonth exemption for barometers". European Parliament. 10 July 2007. Retrieved 2021-05-11 .
- ^ Stanton, William (1975). The Corking Us Exploring Expedition . Berkeley: University of California Press. pp. 126. ISBN0520025571.
- ^ Hood, Jean (5 December 2017). "Barometers : History, working and styles". Retrieved 21 June 2020.
- ^ "Natalo Aiano". Well-nigh us page. C. Aiano & Sons Ltd. 22 May 2017.
- ^ Nicholas, Goodison (1977). English language barometers 1680-1860 : a history of domestic barometers and their makers and retailers (Rev. and enl. ed.). Antiquarian Collectors' Society. ISBN978-0902028524.
- ^ Tomlinson, Stuart (Feb 10, 2013) Large barometer at Portland State University could be the tallest in the world. oregonlive.com
- ^ Figuier, Louis; Gautier, Émile (1867). L'Année scientifique et industrielle. 50. Hachette et cie. pp. 485–486.
- ^ "MEMS Barometric Pressure Sensor". Sensors & Transducers E-Digest. 92 (4). 2008. Retrieved xiii June 2014.
- ^ This Is the Samsung Milky way Nexus, Google's New Official Android Telephone. Gizmodo.com (2011-10-18). Retrieved on 2011-eleven-xv.
- ^ Molen, Brad (2011-ten-20). "Behind the glass: a detailed tour inside the Samsung Galaxy Nexus". Engadget. Engadget. Archived from the original on 2014-12-05. Retrieved 2015-06-23 .
Barometric pressure sensor: BOSCH BMP180
- ^ "BMP180: Digital, barometric pressure sensor" (PDF). Bosch. Archived from the original (PDF) on 2015-06-23. Retrieved 2015-06-23 .
- ^ Milky way Nexus barometer explained, Sam Champion not out of a job. Engadget (2011-10-20). Retrieved on 2011-12-03.
- ^ Muralidharan, Kartik; Khan, Azeem Javed; Misra, Archan; Balan, Rajesh Krishna; Agarwal, Sharad (2014-02-26). "Barometric Phone Sensors – More Hype Than Hope!". ACM HotMobile: ii. Retrieved 2015-06-23 .
- ^ Shark Oil Barometer Archived July twenty, 2011, at the Wayback Machine Barometer World.
- ^ Understanding air pressure. USA Today.
- ^ Using winds and a barometer to brand forecasts. U.s. Today (17 May 2005).
- ^ Hopkins, Edward J. (1996-06-10). "Surface Weather condition Analysis Chart". University of Wisconsin. Archived from the original on 28 April 2007. Retrieved 2007-05-x .
- ^ Pearce, Robert Penrose (2002). Meteorology at the Millennium. Academic Press. p. 66. ISBN978-0-12-548035-2 . Retrieved 2009-01-02 .
- ^ Applying The Barometer To Atmospheric condition Watching. Weather Doctor.
- ^ Report on the Explosion which occurred at the Trimdon Grange Colliery on the 16th February 1882 , retrieved 23 July 2015
- ^ The Encyclopedia of Recreational Diving . Santa Ana, CA, United states of america: Professional Association of Diving Instructors. 1990. pp. 3–96–3–99. ISBN978-1-878663-02-3.
- ^ "Kelvin calibration in depth". Retrieved 12 Feb 2020. [ permanent expressionless link ]
- ^ Berberan-Santos, Yard. N.; Bodunov, E. North.; Pogliani, L. (1997). "On the barometric formula". American Periodical of Physics. 65 (5): 404–412. Bibcode:1997AmJPh..65..404B. doi:10.1119/1.18555.
- ^ Cengal, Yunus A. and Boles, Michael A. (2014) Thermodynamics: An Engineering Approach. McGraw-Colina Educational activity. ISBN 978-0073398174
Further reading
- . Encyclopædia Britannica. Vol. 3 (11th ed.). 1911.
- Burch, David F. The Barometer Handbook: A Modernistic Look at Barometers and Applications of Barometric Force per unit area. Seattle: Starpath Publications (2009), ISBN 978-0-914025-12-2.
- Middleton, W. E. Knowles. (1964). The History of the Barometer. Baltimore: Johns Hopkins Press. New edition (2002), ISBN 0-8018-7154-nine.
External links
![]()
Wikimedia Eatables has media related to Barometers.
-
 Works related to Observations upon the Marine Barometer ... at Wikisource
Works related to Observations upon the Marine Barometer ... at Wikisource
Used To Measure Air Pressure,
Source: https://en.wikipedia.org/wiki/Barometer
Posted by: randallhatione.blogspot.com


0 Response to "Used To Measure Air Pressure"
Post a Comment